Chapter 43
Hypopharynx Cancer
Hiral K. Shah
Deepak Khuntia
Henry T. Hoffman
Paul M. Harari
There is a strong association between tobacco use and the development of hypopharynx cancer (13,59,68). Due to the rich lymphatic network in this anatomic region, patients commonly present with regional nodal metastases. Many hypopharynx cancer patients also carry significant medical comorbidities and social issues that present additional challenges to the successful delivery of aggressive cancer therapy. As for all complex tumors of the head and neck region, multidisciplinary evaluation and management is critical and should involve a head and neck surgeon, radiation oncologist, medical oncologist, nurse, nutritionist, speech/swallow therapist, and social worker. Although a selected cohort of early stage tumors may be amenable to organ preservation surgery, more radical surgery such as laryngopharyngectomy is often required for patients who undergo a primary operative approach for hypopharynx cancer. This ablative procedure can induce significant cosmetic and functional changes, and postsurgical rehabilitation efforts guided by knowledgeable professionals are very important to assist in patient adaptation. Increasingly, hypopharynx cancer patients are being considered for nonoperative treatment approaches using definitive radiation or chemoradiation as a means of obtaining tumor control with preservation of organ function. Regardless of the specific treatment approach, all patients require active rehabilitation therapy in an effort to maximize their ultimate speech and swallow function. Despite stepwise advances in the diagnosis and treatment of hypopharynx cancer, the overall outcome for these patients is relatively poor compared with other head and neck cancer sites. As with most tumors of the head and neck region, there is significant interest in combining molecular targeted therapies with traditional cytotoxic therapy in an effort to further improve outcome.
Anatomy
The hypopharynx, sometimes referred to as the laryngopharynx, is contiguous superiorly with the oropharynx and inferiorly with the cervical esophagus (Fig. 43.1).
As general landmarks, the superior border of the hypopharynx is demarcated by the hyoid bone and the inferior border by the cricoid cartilage. With regard to cancer diagnosis and staging, there are three primary anatomic subsites within the hypopharynx: the bilateral pyriform sinuses, the postcricoid region, and the posterior pharyngeal wall.
The pyriform sinuses are essentially inverted pyramids with the medial, lateral, and anterior walls narrowing inferiorly to form the apices. Posteriorly, the pyriform sinuses are open and contiguous with the pharyngeal walls. Superiorly, the sinuses are surrounded by the thyrohyoid membrane through which passes the internal branch of the superior laryngeal nerve. Tumor involvement of the sensory branches of this nerve can result in referred otalgia. The postcricoid region is comprised of the mucosa overlying the cricoid cartilage, with the arytenoid and esophageal mucosa forming the superior and inferior borders, respectively. The posterior pharyngeal wall is predominantly comprised of squamous mucosa covering the middle and inferior pharyngeal constrictor muscles and is separated from the prevertebral fascia by the retropharyngeal space. Typically, the mucosa lining the pharyngeal wall is <1 cm in thickness and provides a minimal barrier to direct tumor infiltration. The posterior pharyngeal wall is contiguous with the lateral wall of the pyriform sinus (Fig. 43.2A,B).
Sensory innervation of the hypopharynx is provided by the internal branch of the superior laryngeal nerve as well as fibers deriving from the glossopharyngeal nerve. The recurrent laryngeal nerve and the pharyngeal plexus provide the primary motor supply. The arterial supply of the hypopharynx is derived primarily from branches of the external carotid artery: superior thyroid arteries, ascending pharyngeal arteries, and lingual arteries.
There is a rich network of lymphatics within the hypopharynx that drain directly through the thyrohyoid membrane and into the jugulodigastric lymph nodes, most commonly involving the subdigastric node. Additionally, there may be direct drainage into the spinal accessory nodes. Tumors involving the posterior pharyngeal wall can also drain into the retropharyngeal nodes, including the most cephalad retropharyngeal nodes of Rouviere.
Epidemiology and Etiology
Hypopharynx cancers are relatively uncommon. Approximately 2,500 to 3,000 cases are diagnosed annually in the United States. The pyriform sinus is the most common subsite of origin comprising 65% to 75% of hypopharynx cases (15). Although it is sometimes difficult to definitively assign tumor origin to a single subsite, approximately 10% to 20% of hypopharynx tumors arise from the posterior pharyngeal wall and 5% to 15% originate from the postcricoid region (5). Due to the rich lymphatic drainage of the hypopharynx, at least 50% of patients will manifest clinically positive cervical lymph nodes at the time of diagnosis (43). Jugular chain nodes, levels II through IV, as well as retropharyngeal nodes, are all at high risk to harbor regional metastases in patients with hypopharynx cancer. In parallel to cancers of the nasopharynx, retropharyngeal nodes may be the first site of nodal spread. Postcricoid tumors may also spread directly to pre- and paratracheal nodal basins. Due to the high propensity for advanced primary disease as well as regional nodal involvement, the majority of hypopharynx cancer patients present with stage III and IV disease. In a retrospective study from Washington University, 87% of patients with cancers of the pyriform sinus and 82% of patients with posterior pharyngeal wall tumors presented with stage III or IV disease (65).
Over 90% of patients with hypopharynx cancer report past cigarette use (40). Alcohol appears to potentiate the carcinogenic effects of tobacco but in isolation is not clearly associated with an increased risk of developing hypopharynx cancer. The index hypopharynx cancer often occurs within a field of diseased mucosa characterized by high-grade dysplasia. This “field cancerization” reflects widespread mucosal exposure to carcinogens and is responsible for the high rate of synchronous and metachronous primary tumors identified in patients with hypopharynx cancer. Successful counseling with particular emphasis on smoking cessation can enhance treatment tolerance and diminish the risk of developing subsequent cancers of the upper aerodigestive tract. Patients with occupational exposure to coal dust, steel dust, iron compounds, and fumes have also shown an increased risk for developing hypopharynx cancer (6,60). Overall, the incidence of hypopharynx cancer has shown some gradual decline in the United States. From 1975 to 2001 the incidence decreased by approximately 35%, perhaps as a result of smoking cessation efforts (21).
Human papilloma virus (HPV) infection is well established as a risk factor for the development of squamous-cell carcinoma of the gynecologic tract, particularly in the uterine cervix. The relationship between HPV and head and neck cancer is only recently becoming better appreciated, particularly for cancers of the nasopharynx and oropharynx. Studies have demonstrated that approximately 20% to 25% of patients with hypopharynx cancer test positive for HPV DNA (42,50). Currently, the clinical implications of the presence of HPV in hypopharynx cancer are yet to be defined.
There is a recognized increased risk of developing cancers of the postcricoid region for patients with Plummer Vinson syndrome, characterized by iron-deficiency anemia, hypopharyngeal webs, weight loss, and dysphagia (75). Favorable changes in the epidemiology of hypopharynx cancer have resulted from changes in nutrition. The addition of iron to flour has made Plummer Vinson syndrome quite rare in the upper midwestern United States and Scandinavian countries where it was formerly more common. An associated decrease in hypopharynx cancer involving the postcricoid region has followed.
Prognostic Factors
Several prognostic factors have been identified for patients with hypopharynx cancer. Age, particularly >70 years, has been identified as an unfavorable predictor of outcome (24). This may simply reflect the diminished likelihood of elderly patients to successfully tolerate the aggressive therapy approaches required for locoregionally advanced cancers of the head and neck. Women have been found to achieve somewhat improved outcomes compared to men, although this may in part be a manifestation of earlier stage disease at diagnosis (63,64). In addition, tumor location has an impact on outcome with cancers of the pyriform sinus generally faring better than those arising in the postcricoid or posterior pharyngeal wall regions (63,64). As a whole, hypopharynx cancer patients fare poorly in comparison with patients harboring tumors from other head and neck sites. To a lesser extent, tobacco, alcohol, and dietary factors (carotenoids, vitamin C, vitamin E, and flavonoids) may also have an impact on outcome (23).
Biologic factors have been investigated for their potential role in hypopharynx cancer. The presence of p53 gene mutations have been associated with bulkier tumors and younger patients along with higher expression of the epidermal growth factor receptor (EGFR). However, p53 has not shown correlation with multiple primary tumors, tumor grade, or DNA ploidy (15,28). Further, definitive data in hypopharynx cancer does not exist correlating a prognostic significance of EGFR expression with overall outcome (29).
Staging
The most commonly used system for staging hypopharynx cancer is the American Joint Committee on Cancer (AJCC) 2002 edition of their staging manual, which is based on a combination of clinical and radiographic data (Table 43.1) (30). The nodal and group staging is similar to other sites within the pharynx with the exception of nasopharynx. One must always exercise good clinical judgment when designing treatment recommendations based on AJCC staging. With specific regard to hypopharynx cancer, the AJCC staging system does not differentiate the specific tumor subsite or number of walls invaded by tumor that may have prognostic significance as well as management implications. Moreover, patient factors including age, comorbid medical conditions, and motivation for organ preservation are beyond the scope of the staging system, but nevertheless represent important factors for consideration with each individual patient.
Patterns of Spread
Local Extension
Cancers arising from the pyriform sinus may spread superiorly to involve the aryepiglottic folds and arytenoids and invade the paraglottic and pre-epiglottic space. Lateral tumor extension can involve portions of the thyroid cartilage, allowing entry into the lateral compartment of the neck. High-resolution computed tomography (CT) or magnetic resonance imaging (MRI) is often useful for optimal assessment regarding the extent of tumor invasion. For tumors arising from the medial wall, the most common site of involvement for pyriform sinus tumors, there is a likelihood of tumor involvement of intrinsic muscles of the larynx resulting in vocal cord fixation. Inferior tumor extension beyond the apex can involve the thyroid gland.
Cancers arising within the postcricoid region can extend circumferentially to involve the cricoid cartilage or anteriorly to involve the larynx with resultant vocal cord fixation. Tumor involvement of the recurrent laryngeal nerve can also precipitate vocal cord fixation. Primary postcricoid tumors are often quite extensive and can involve the pyriform sinus, trachea, or esophagus. As a result, these tumors generally carry a worse prognosis in comparison to tumors from other subsites of the hypopharynx (66). Nodal spread to the paratracheal nodes and inferior deep cervical nodes is not uncommon. Tumor arising from the posterior pharyngeal wall can extend to involve the oropharynx superiorly, the cervical esophagus inferiorly, and the prevertebral fascia and retropharyngeal space posteriorly.
Many cancers of the hypopharynx have a propensity for submucosal spread. It can therefore be difficult to accurately quantify the full microscopic extent of disease. This is particularly true for cancers of the posterior pharyngeal wall and postcricoid regions. Careful study through serial sectioning of surgical specimens has identified that 60% of hypopharynx cancers demonstrate subclinical spread with a range of 10 mm superiorly, 25 mm medially, 20 mm laterally, and 20 mm inferiorly (39). This extensive pattern of tumor infiltration can present considerable challenges in the effort to achieve clear surgical margins or full dosimetric coverage with radiotherapy.
Regional Disease
Lymphatics of the pyriform sinus can drain through the thyrohyoid membrane, across the pretracheal nodes, and into level II and III cervical nodes (Table 43.2). Tumors arising from the posterior pharyngeal wall can involve the retropharyngeal nodes (Rouviere's nodes) extending cephalad to the base of skull. In light of cross-draining lymphatics, there is a significant risk of bilateral cervical adenopathy associated with cancers arising in the hypopharynx (51) (Fig. 43.3).
Distant Metastases
The most common site for distant metastasis to develop in patients with cancer of the hypopharynx is the lung. Approximately one quarter of patients diagnosed with hypopharynx cancer either present with distant metastasis or develop them at some time during the course of their disease (48). For those patients not rendered free of locoregional disease following initial therapy, the incidence of distant metastases increases notably with the length of time following initial treatment (45).
Field Cancerization
Carcinogens can induce dysplastic changes throughout the mucosa of the upper aerodigestive tract, leading to an increased risk for field cancerization that enhances the likelihood of synchronous or metachronous secondary primary tumors. Approximately 7% of patients with hypopharynx cancer will manifest a secondary primary tumor at initial diagnosis and between 10% to 20% will develop a secondary primary tumor over time. In fact, this second tumor risk is a significant cause of mortality in patients who survive >2 years following initial treatment (38).
Clinical Presentation
In light of the nonspecific nature of early symptoms, the majority of patients with cancers of the hypopharynx present with advanced local and/or regional disease. Frequently, there is a delay between presentation and diagnosis as patients are often managed for presumed infectious or gastrointestinal etiology. The majority of symptoms are related to local tumor spread including dysphagia and odynophagia. There may be frank esophageal obstruction, invasion of constrictor muscles, prevertebral space invasion, or strap muscle invasion. Common presenting signs and symptoms include dysphagia, sore throat, hoarseness, weight loss >10 pounds, and neck mass. The majority of patients present with more than one of these signs and symptoms (70). Selected patients may first come to medical attention with complaints of unilateral ear pain (referred otalgia) due to tumor involvement of the nerve of Arnold, a branch of the superior laryngeal nerve.
A comprehensive work-up for patients with cancers of the hypopharynx should include a detailed history focusing on the duration of symptoms, amount of weight loss, the presence of otalgia, changes in voice quality, and degree of dysphagia. Factors such as previous history of an upper aerodigestive tract malignancy and smoking are important. A detailed physical examination should include direct and indirect visualization of the full laryngopharyngeal axis with particular attention to the size, location, and anatomic positioning of the primary tumor as well as the mobility status of the true vocal cords. Dentition and oral health should be assessed. If the patient presents with cervical adenopathy, the size, number, location, texture, and mobility of these nodes should be documented.
Although cervical adenopathy associated with hypopharynx cancer may be analyzed with fine needle aspiration biopsy, there is little value in this approach for most patients who will undergo a direct laryngoscopy under general anesthesia in conjunction with esophagoscopy. This panendoscopy permits not only biopsy confirmation of the primary tumor site, but also mapping of the extent of the tumor as well as survey for synchronous primary tumors (Fig. 43.4).
Staging Work-Up
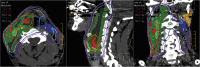
In addition to panendoscopy, patients should undergo either high-resolution CT with contrast (or MRI) extending from skull base to below the clavicle to help assess the extent of the primary tumor and to quantitatively and qualitatively assess cervical adenopathy (53) (Fig. 43.5 and 43.6).
Patients with cancer of the hypopharynx should also undergo formal swallow evaluation to assess their functional swallow capacity before the initiation of therapy. This analysis may be done as a “bedside” study of swallowing capacity, a fiberoptic endoscopic swallowing evaluation (FEES), or through the more definitive fluoroscopic barium swallow study. This modified barium swallow study is called a cookie swallow, videopharyngogram, or oropharyngeal motility study (OPMS). Either a chest x-ray or CT is recommended to assess the presence of pulmonary metastasis.
F-18-deoxyglucose or fluorodeoxyglucose/positron emission tomography (18FDG-PET) imaging is increasingly used to help assess the extent of the primary tumor and regional adenopathy as well as the presence of distant metastasis. FDG-PET is becoming an increasingly valuable adjunct to CT and/or MRI in the radiation treatment planning process, particularly for patients treated with conformal IMRT or tomotherapy techniques. Di Martino et al. (22) compared CT, PET, color-coded duplex sonography, palpation, and panendoscopy in assessment of tumor and nodal status. The results of this study are summarized in Table 43.3 and support the promising sensitivity and specificity of PET scanning in head and neck cancers. Schwartz et al. (59) examined standardized uptake value (SUV) of primary and nodal metastasis in head and neck cancer patients and their relationship to clinical outcome. A primary tumor SUV >9.0 was associated with a significantly lower local recurrence-free survival and disease-free survival (DFS). There was no correlation between nodal SUV and clinical outcome.
Pathological Classification
Review of the National Cancer Data Base (NCDB) Benchmark Reports identified 3,519 cases of hypopharynx cancer reported from 1,542 hospitals during 2000 and 2001. Over 93% of the cases were reported as squamous-cell carcinoma with “other specified types” representing 6.9% of cases (53). On rare occasion, lymphoma, sarcoma, adenocarcinoma, or adenoid cystic carcinoma may present in the hypopharynx, but each histology makes up <0.5% of overall hypopharynx cancer diagnoses (21). Pretreatment Evaluation Many patients with hypopharynx cancer present with concurrent medical and social comorbidities that require consideration before initiating cancer-directed therapy. Commonly, there is a progressive history of dysphagia and odynophagia with associated weight loss. Whether these patients are treated with surgical or nonsurgical approaches, a gastrostomy tube may need to be considered as a temporary measure. It is important to optimize or at least stabilize the patient's nutritional status prior to initiating definitive therapy. It is valuable for hypopharynx cancer patients to undergo evaluation by a speech and swallow therapist to evaluate the degree of dysfunction prior to therapy. Patients may be able to use adaptive techniques to improve the effectiveness and safety of their oral intake. Additionally, close follow-up with the same speech and swallow therapist is highly desirable during and after therapy to maximize the patient's long-term functional capabilities. Since many hypopharynx patients have an active history of alcohol and tobacco use, it is important to counsel accordingly and encourage all patients to take advantage of methods and programs to facilitate smoking and alcohol cessation. All patients should undergo comprehensive dental evaluation and cleaning as well as basic education regarding oral hygiene. For patients treated with conventional radiation therapy techniques there is a significant likelihood of long-term xerostomia that can promote dental decay. If existing dentition is in poor condition, dental extractions should be considered prior to therapy, particularly for teeth that will reside within the high-dose radiation region. Typically 10 to 14 days are required following dental extractions to allow for healing prior to the initiation of radiation therapy. Custom fluoride carrier trays should be fabricated and discussed for long-term use in an effort to diminish the rate of dental decay for patients with chronic xerostomia. Finally, many patients with hypopharynx cancer will have social issues including lack of family support, financial limitations, transportation issues, poor nutrition, and hygiene habits that may hamper their ability to successfully receive adequate care. Often, the involvement of a case manager or social worker is of central importance to assist patients who require support both during as well as following cancer therapy. Management T1–2 Tumors Surgery Contemporary indications for primary surgical management of patients with early cancers of the hypopharynx include those with a history of previous head and neck radiation, those in whom organ conservation approaches are deemed possible (a relatively small proportion of cases), and those who refuse radiation. Even for hypopharynx cancer patients who will receive nonoperative treatment approaches, it remains critical for the head and neck surgeon to remain actively involved. The role of the surgeon in these cases may include endoscopic biopsy with detailed assessment of tumor extent, methods to secure the airway (tracheotomy or laser debulking), and methods to ensure adequate nutrition (gastrostomy). Patients with hypopharynx cancer also require careful evaluation regarding regional nodal metastases. For N0–1 patients treated with primary radiation or chemoradiation approaches, adjuvant neck dissection is generally unnecessary. However, for patients presenting with N2–3 neck disease, careful evaluation of tumor response in the neck is important to help gauge the potential value of adjuvant neck dissection following radiation or chemoradiation. Although an increasing number of reports suggest that detailed imaging of the neck 8 to 12 weeks postradiation with FDG-PET can serve as a valuable guide to help select those patients warranting adjuvant neck dissection, many institutions mandate adjuvant neck dissection for all patients presenting with N2–3 neck disease in an effort to maximize regional disease control. Both approaches are readily defendable at present (10,56,67,68,74). If neck dissection is performed, this provides an opportunity for the surgeon to reassess the primary tumor site under anesthesia with directed biopsy if suspicious for residual disease. If residual disease at the primary site is highly suspected or confirmed by biopsy several months following completion of radiation or chemoradiation, this will prompt consideration regarding the feasibility and advisability of salvage surgery options. A selected cohort of T1 and T2 hypopharynx cancers may lend themselves to surgical excision. These favorable subsites include the upper pyriform sinus and the posterior pharyngeal wall. The standard supraglottic laryngectomy encompasses the aryepiglottic fold and may be extended to include part of the arytenoids, base of tongue, and upper pyriform sinus. Small cancers isolated to the posterior pharyngeal wall may be removed by endoscopic laser resection or removal using an open approach. Dysphagia requiring no food by mouth status is common from the open approach, especially if reconstruction of the posterior wall is effected with an adynamic and insensate free flap. Relative contraindications to organ conservation surgery for hypopharynx cancers include transglottic tumor extension, cartilage invasion, vocal fold paralysis, postcricoid invasion, deep pyriform sinus invasion, and extension beyond the larynx. Innovations with free flap reconstruction have permitted retention of speech and swallowing and breathing functions of the larynx despite extensive resection by way of a hemilaryngopharyngectomy. The temporoparietal flap and radial forearm free flap coupled with rigid cartilaginous support have been employed to retain function in patients with hypopharynx cancers without extension to the postcricoid region or apex of the pyriform sinus (58,71). Radiation Curative radiation therapy (RT) is generally the preferred treatment option for patients with T1–2 hypopharynx tumors. This approach affords good potential for organ preservation without compromise in clinical outcome. A classical course of radiation therapy for hypopharynx cancer lasts 6 to 7 weeks, with treatment delivered 5 days per week. Conventional treatment involves a shrinking field technique that initiates with opposed lateral fields encompassing the primary tumor and upper neck lymphatics with a matched anterior field to complete treatment of the lower neck. One of the most common worldwide fractionation regimens involves the delivery of 2 Gy daily fractions to 70 Gy over 7 weeks. Altered fractionation techniques including hyperfractionation (e.g., 1.1 to 1.4 Gy twice daily) and accelerated fractionation (e.g., six fraction per week or concomitant boost regimens) have demonstrated improved locoregional control rates for head and neck cancer patients (31,54,61). A recent meta-analysis examined 15 trials that compared conventional fractionation to altered fractionation, either hyperfractionation or accelerated fractionation. The study demonstrated a small but statistically significant survival benefit of 3.4% at 5 years with altered fractionation. The benefit was higher with hyperfractionation compared to accelerated fractionation and was more pronounced for patients younger than age 50 (9). Due to the high likelihood of subclinical nodal metastases, even in the clinically N0 neck, patients traditionally receive comprehensive radiation to encompass nodal regions from skull base to clavicle. Due to the varying thickness of the head and neck, custom compensators or wedges should be used for the lateral fields to obtain a more homogeneous dose distribution. Shrinking field techniques to spare direct spinal cord dose after ~45 Gy, as well as final mucosal field reductions after 54 to 60 Gy are often appropriate with posterior neck boosting with electrons to complete the nodal dosing without excessive dose to the spinal cord. Over the past 10 to 15 years, the use of three-dimensional CT-based planning has become routine in the management of head and neck cancer patients (Fig. 43.7).
CT-based planning allows precise delineation of target volume and visualization of dose distributions (Fig. 43.8).
Early T-stage hypopharynx patients with N0–1 neck disease can be considered for treatment with radiation alone or concurrent radiation plus chemotherapy. In this setting, gross disease should receive 70 Gy and the contralateral neck (N0) should receive 50 to 54 Gy. With T1-N0 lesions, patients may achieve 5-year disease-specific survival (DSS) on the order of 90%, while T2-N0 lesions may achieve DSS of >70% (52). (Tables 43.4 and 43.5).
Patients with advanced N2–3 neck disease are often considered for postradiotherapy or postchemoradiotherapy neck dissection in an effort to maximize the likelihood of neck control (47). Several recent studies, including one from the University of Iowa, have assessed the value of a postradiation FDG-PET to help select those patients who might benefit most from subsequent neck dissection. For complete clinical responders, the Iowa study concluded that FDG-PET in this setting has a very high negative predictive value. The authors suggest that FDG-PET may be a valuable tool to help determine which patients should undergo adjuvant neck dissection versus observation following the completion of head and neck radiation or chemoradiotherapy (79).
Squamous-cell carcinomas of the head and neck are rapidly proliferating tumors. There has been significant interest over the past several decades in the use of intensified radiation fractionation schedules to counter rapid tumor cell repopulation as a means of improving outcome in head and neck cancer patients treated with radiation alone. The Radiation Therapy Oncology Group (RTOG 9003) altered fractionation in a randomized trial comparing conventional fractionation to hyperfractionation, split-course, and concomitant boost techniques and demonstrated a significant improvement in DFS for the hyperfractionation and concomitant boost arms (31) (Table 43.6). Hypopharynx patients were included within the study cohort for this randomized trial. These altered fractionation regimens were associated with higher incidence of grade 3 or worse acute mucosal toxicity but no significant difference in overall chronic toxicity at 2 years following completion of treatment.
In the past several years, there has been significant interest in the use of intensity-modulated radiation therapy (IMRT) in head and neck cancer as a means of diminishing normal tissue toxicities, particularly xerostomia resulting from irradiation of major salivary glands. Excellent candidates for IMRT include patients with unilateral T1–3 primary lesions with ≤N2b neck disease. In light of the high-dose gradients that can accompany highly conformal plans, a critical component of successful IMRT delivery is the use of an accurate and reproducible localization system. At several centers, the use of an optically guided localization system is used to enhance treatment precision for patients undergoing IMRT for head and neck cancer (41) (Fig. 43.9).
The cephalad margin of the N0 contralateral neck may often be limited to the C1–2 interspace in an effort to further improve parotid gland sparing (24,25).
T3–4 Resectable
Surgery
Favorable T3 hypopharynx cancers that present in the upper aspect of the pyriform sinus and permit full extirpation by either an extended supraglottic laryngectomy or extended vertical partial laryngopharyngectomy with free flap reconstruction are possible but infrequent. Many T3 and T4 hypopharynx cancers that are treated surgically will require total laryngectomy with efforts to preserve a posterior strip of the hypopharynx spanning the oropharynx to the esophagus. This preserved posterior wall of the hypopharynx may be tubed and closed on itself in selected cases. In the past it was common practice to accept primary reconstruction of this segment as adequate for swallowing with closure over a nasogastric tube. More recently primary closure has been discouraged for cases with less than a 3 to 3.5 cm width of posterior pharyngeal wall mucosa to tube on itself. Most commonly superior swallowing results when the anterior and lateral walls of the remaining hypopharynx are reconstructed with either pedicled or free flap reconstruction.
For more bulky tumors of the hypopharynx, total laryngopharyngectomy is required and refers to removal of the larynx and the entire hypopharynx. This procedure creates a gap between the oropharynx and esophagus that can be reconstructed with a tubed fasciocutaneous flap such as the radial forearm free flap or lateral thigh flap, a free jejunum, or a tubed pedicled myocutaneous flap. The myocutaneous flaps are technically difficult to tube due to the bulk of the fat and muscle underlying the skin paddle.
Laryngopharyngectomy with esophagectomy may be performed if the hypopharynx cancer extends inferior to the cricopharyngeus to ensure the inferior margin. In this case, reconstruction with a gastric pull-up or colon interposition are options used to restore the conduit for food and saliva extending from the oropharynx to the stomach.
Postoperative Radiation Therapy
In light of the deeply infiltrative nature of advanced hypopharynx cancers that are treated with initial surgical resection, the vast majority of patients are recommended to undergo adjuvant radiation therapy in an effort to enhance locoregional control rates. Classical indications for postoperative radiation include T4 primary tumors, close or positive microscopic margins, cartilage/bony invasion, >1 metastatic lymph node, or the presence of extracapsular extension (ECE). Conventional therapy involves the use of shrinking field techniques, as described previously, to deliver 54 to 63 Gy to all areas at risk and a boost to 60 to 66 Gy to regions of ECE and/or positive margins. The entire cervical nodal chain from the skull base to the clavicle bilaterally should be included. IMRT techniques may be considered in an attempt to reduce radiation dose to normal tissue structures such as the contralateral parotid gland and thereby preserve better salivary function.
Recently, the role of concurrent chemotherapy along with postoperative radiation has been evaluated in prospective randomized trials by the RTOG and European Organization for Research and Treatment of Cancer (EORTC). Eligibility criteria in the RTOG trial included patients with two or more positive nodes, ECE, or microscopically positive margins. All patients received 60 Gy alone or with concurrent cisplatin 100 mg/m2 every 3 weeks. This trial demonstrated an improvement in locoregional control and DFS for patients who received concurrent chemoradiotherapy. However, no significant benefit in absolute survival was confirmed (Table 43.7) (20). The EORTC conducted a similar trial that included patients with stage III (except T3 N0 larynx), stage IV, and patients with stage I or II with positive margins, lymphovascular invasion, and perineural invasion. All patients received 66 Gy alone or with cisplatin at 100 mg/m2 every 3 weeks. This trial demonstrated a significant improvement in progression-free survival and overall survival with the addition of chemotherapy (6) (Table 43.8).
Although the studies above identify that the addition of cisplatin chemotherapy to postoperative radiation can improve tumor control outcome for specific categories of high-risk patients, it is clear that this modest benefit comes at the expense of additional toxicity. Careful clinical judgment regarding the selection of patients most likely to tolerate and thereby benefit from this approach is warranted. In the definitive treatment setting, there is mounting evidence that patients >70 years of age derive little to no benefit from the addition of systemic chemotherapy to radiation in head and neck cancer (9,55). This is quite likely to be true in the postoperative head and neck cancer treatment setting as well. The inadvertent introduction of treatment breaks during the adjuvant radiation course can easily compromise the potential benefits of the combined modality therapy in this setting.
There is considerable interest in the use of molecular targeted therapies in the treatment of head and neck cancer patients. The most mature clinical data set in head and neck cancer involves the use of EGFR inhibitors such as cetuximab (monoclonal antibody against the EGFR). An international phase III trial comparing high-dose radiation alone versus radiation plus cetuximab in advanced head and neck cancer patients confirmed a locoregional control improvement (10% at 3 years) and overall survival advantage (10% at 3 years) with the addition of cetuximab (7). A relatively small subset of patients with hypopharynx cancer were enrolled in this study of 424 patients, and this subset did not demonstrate a clear advantage with use of the EGFR inhibitor treatment. Ongoing trials to examine the potential value of adding cetuximab to concurrent chemoradiation approaches in advanced head and neck cancer are in progress in both the definitive and high-risk postoperative settings.
Definitive Radiation Therapy
There are several reasons why hypopharynx cancer patients who are technically resectable may not undergo primary surgery. These include age (e.g., patients >70 or 80 years old), the presence of significant medical comorbidities and/or patient unwillingness to accept total laryngectomy. Curative-intent radiation or chemoradiation is often pursued in these settings. Conventional radiation therapy commonly involves a shrinking three-field technique to deliver ~70 Gy in 2 Gy daily fractions to areas of gross disease and 50 to 60 Gy to areas of microscopic disease. If patients are scheduled to undergo postradiotherapy neck dissection, then gross nodal disease can be limited to 60 to 63 Gy. If patients are not candidates for postradiotherapy neck dissection, then gross nodal disease should be carried to 70 Gy. Altered fractionation regimens such as hyperfractionation or accelerated fractionation should be considered for patients being treated with radiation alone, as this approach has been demonstrated to improve the likelihood of locoregional tumor control (31).
In patients with adequate performance status, concurrent chemoradiation strategies using platinum-based chemotherapy should be considered. The most comprehensive meta-analysis to examine the benefit of chemotherapy in advanced head and neck cancer confirms a small but significant survival advantage for the use of chemotherapy, with the best gains observed with the use of concurrent platinum-based regimens (8%) (9,55).
However, this meta-analysis also confirms a steadily decreasing benefit for the use of chemotherapy with advancing patient age, such that no advantage is observed for patients >70 years of age. This same loss of statistical benefit for patients >70 years of age is also observed for the outcome gains derived from altered fractionation over conventional fractionation. Therefore, once daily radiation regimens (conventional technique or IMRT) may be quite reasonable for hypopharynx patients >70 years of age (or selected patients with modest performance status) rather than intensified fractionation regimens or the use of concurrent chemotherapy.
Recently, there has been renewed interest in the concept of induction chemotherapy approaches for patients with locoregionally advanced head and neck cancer, particularly with the introduction of taxane-containing regimens that offer promise to improve tumor response rates. Two randomized trials have been reported that compare induction 5-fluorouacil (5-FU) and cisplatin versus 5-FU, cisplatin, plus a taxane (37,73). Preliminary reports suggest a significant improvement in overall response rate with the addition of a taxane. In an effort to simultaneously enhance locoregional disease control and reduce distant metastases, several phase III trials are in progress that compare this sequential approach (triple agent induction chemotherapy followed by concurrent chemoradiation) versus concurrent chemoradiation (current standard of care) for patients with locoregionally advanced head and neck cancer (1). These aggressive approaches certainly appear worthy of controlled clinical investigation for head and neck subsites such as the hypopharynx, where the overall outcomes are poor and both locoregional control and distant metastases present a formidable challenge. Nevertheless, maturation of these trials is important before the ad hoc adoption of such complex, costly, and toxic treatment strategies. Careful assessment of tumor control, survival, and long-term functional outcome dovetailed with quality of life evaluation will be important to help place these regimens in the best perspective for advanced head and neck cancer patients.
Management of hypopharynx cancer has gradually evolved over the past decades to reflect the steady advancement of nonsurgical therapy. Data from the NCDB Benchmark reports addressing 3,519 cases diagnosed in 2000 to 2001 reveals the combination of radiation and chemotherapy to be the most common initial treatment overall (32.5%) for all stages of hypopharynx cancer (Fig. 43.10).
Radiation as a single modality therapy was the most common initial treatment for stage I hypopharynx cancer (34%) followed by surgery alone (19.4%) as next most common (Fig. 43.11A). Chemoradiation was the most common treatment for stage II (34.4%), stage III (37%), and stage IV (35.7%) disease (Figs. 43.11B–D) (53).
Unresectable, Nonmetastatic Disease
The management of patients with unresectable locoregional disease without distant metastases is dependent on patient performance status. A patient with a good performance status may be offered definitive radiotherapy or concurrent chemotherapy as discussed above. In a randomized Intergroup trial of unresectable head and neck cancers, the addition of high-dose cisplatin to radiation was found to improve survival versus radiation alone, although at the expense of increased toxicity (2) (Table 43.9). However, patients with poor performance status who are not considered candidates for aggressive radiation or chemoradiation approaches should be managed with palliative intent. This may include short-course radiation regimens such as 4 to 5 Gy of five fractions over 1 to 2 weeks with a repeat of the same 3 weeks hence if favorable initial tolerance and response is achieved. Systemic chemotherapy alone can be considered, although for poor performance status patients, best supportive care with medical therapy and airway control may also be appropriate.
P.969
Metastatic Disease
As many as one quarter of hypopharynx cancer patients will develop metastatic disease at some point in their clinical course. In this setting, treatment is palliative and should be delivered to maximize or help maintain quality of life. If patients are having difficulty with local pain, bleeding, or swallowing, palliative short-course radiation therapy can be delivered as described above. Surgery may also provide a reasonable palliative option for selected patients who have incurable disease but significant symptoms related to their localized disease. If aspiration of secretions (despite no food by mouth status and enteral feedings) persists, laryngopharyngectomy may afford a reasonable option to discuss with the patient and family members. Similarly, complete stenosis of the pharynx or upper esophagus due to tumor (or following treatment) may leave a patient with constant need for suctioning his or her own secretions. In selected patients, laryngopharyngectomy with gastric pull-up may be a reasonable palliative option. Finally, G-tube placement can be considered for patients who do not wish to pursue palliative radiation therapy or surgery. Many patients in this setting will benefit from narcotic analgesics for pain management.
Patients with adequate or good performance status should be considered for palliative chemotherapy. Several agents have shown response for recurrent and metastatic (head and neck) cancer including cisplatin, carboplatin, 5-FU, methotrexate, docetaxel, or combination regimens based on platinum or taxane and the more recent introduction of molecular targeted therapies including the EGFR inhibitors (17,19). A randomized trial has been reported comparing the efficacy of cisplatin alone compared with two multiagent regimens: (a) cisplatin and 5-FU and (b) cisplatin, 5-FU, bleomycin, and vincristine. Although the combination regimens demonstrated higher tumor response rates, this did not translate into a significant difference in median survival between the three arms. The combination arms were more toxic (16). In the 1990s there was significant interest in incorporating taxanes into regimens for recurrent and metastatic head and neck cancer. A randomized trial comparing cisplatin and 5-FU to cisplatin and paclitaxel demonstrated similar response rates, median survival, and 1-year survival. The cisplatin and 5-FU arm was more toxic to administer (33). There has also been significant interest in incorporating targeted therapies such as the EGFR inhibitors for head and neck cancer patients with metastatic or recurrent disease. These agents generally elicit modest response rates when given as single agents. For example, cetuximab, gefitinib, and erlotinib have generated response rates of 13%, 11%, and 4%, respectively as single agents in head and neck cancer (18,62,72). There is interest in combining traditional cytotoxic chemotherapy agents with targeted agents to improve overall outcomes. A trial comparing cisplatin alone or in combination with cetuximab demonstrated improved response rates with cetuximab, but no significant improvement in progression-free survival or overall survival (13).
Complications
Surgery
The complications from surgery generally fall within the confines of bleeding, infection, reaction to the anesthesia, and damage to structures around or in the field of surgery. The damage to the laryngopharynx that occurs in the course of removing those tissues involved by cancer necessarily interferes with key laryngeal functions: breathing, swallowing, and speaking.
If an effort is made to preserve laryngeal function, some compromise may be required. A long-term tracheotomy, no food by mouth status with the use of gastrostomy feedings, and/or significant dysphonia are not uncommon for patients with hypopharynx cancer treated with conservation laryngeal surgery. These same complications may attend the more comprehensive laryngopharyngectomy as well. Stenosis of the neopharynx, difficulty with alaryngeal speech, and stomal stenosis may compromise the same functions ordinarily ascribed to the larynx. For all open surgical approaches, the risk of a salivary fistula is greatest for those patients previously treated with radiation. Although salivary fistulas are rare with endoscopic approaches, they have occurred in cases requiring aggressive laser resection.
Radiation Therapy
During a course of head and neck radiation therapy, there are predictable side effects that are experienced by the majority of patients: mucositis, fatigue, loss of taste acuity, radiation dermatitis, and xerostomia. Typically patients will begin to experience mucositis during the 3rd week of radiotherapy. This initially manifests as mucosal blanching within the treatment field, but can progress to patchy or confluent mucositis. Initially patients can be treated with an over-the-counter pain reliever, but once patients develop grade II or III mucositis, they will commonly require narcotic analgesics for adequate pain control. The combination of dysphagia and mucositis can result in significant nutritional compromise, necessitating intravenous hydration and parenteral nutritional supplementation. Nausea associated with treatment can also further complicate the nutritional status. These acute toxicities can become particularly pronounced in the setting of intensified radiation fractionation schedules and/or combined chemoradiotherapy. Patients may require prophylactic antiemetics. In patients receiving concurrent radiotherapy and platinum-based chemotherapy, there is clear potential for myelosuppression; therefore, blood counts should be monitored regularly. Signs or symptoms of infection should be addressed promptly. Finally, xerostomia can become problematic during the course of radiation. Ultimately, patients can be reassured that the majority of these side effects, with the exception of xerostomia, are temporary and will resolve several weeks to months following completion of therapy.
As noted, one of the acute side effects of radiotherapy that can become permanent is xerostomia. Chemical and physical modifiers of the radiation response have been utilized to reduce long-term xerostomia. The free-radical scavenger amifostine has the potential to reduce radiation effects on normal tissues if administered just prior to each radiation fraction. A randomized phase III trial demonstrated a reduction in the severity of the acute and chronic grade 2 or higher xerostomia in patients who received amifostine during RT (11). Dose-limiting toxicities commonly include hypotension and nausea. However, more recent reviews have called into question the ultimate value of amifostine in patients with advanced head and neck cancer, and currently there is no universal standard recommendation across treatment centers for the use of this radioprotector (40,76).
IMRT or tomotherapy techniques allow the clinician to physically modify the radiation dose distribution in an effort to spare critical normal tissues. This approach has been used increasingly for (head and neck) cancer patients to reduce radiation dose to the major salivary glands. A dosimetric analysis comparing radiation dose to the parotid gland and postradiation salivary function demonstrates that limiting mean dose to the parotid gland to <26 Gy is associated with improved postradiation salivary function (25).
In some cases, hypopharynx cancer patients who complete a course of radiation therapy will be noted to have persistent laryngeal edema on subsequent follow-up visits. Although in the early posttreatment phase (in fact up to 24 months), significant or newfound edema should raise suspicion regarding the possibility of persistent or recurrent disease, the majority of patients who receive high-dose radiation across major segments of the larynx and hypopharynx will manifest some degree of edema, mucosal congestion, and eventual fibrosis (see Fig. 43.4B). Generally, this collateral damage is a tolerable chronic toxicity with modest impact on patient quality of life. However, in approximately 10% to 15% of patients, this edema is severe enough to cause significant airway and swallow function compromise requiring tracheostomy.
Outcomes
There are several institutional reports of radiation therapy alone in the management of hypopharynx cancer. It is difficult to compare directly the results between surgically treated patients and radiation treated patients because there is often a selection bias whereby some patients are selected for surgery and others referred for radiotherapy. The University of Florida has systematically reported their results with radiation alone for patients with hypopharynx cancer (Tables 43.10, 43.11 and 43.12) (3,27).
In an effort to examine the potential for organ preservation in patients with advanced cancers of the hypopharynx, the EORTC conducted a randomized trial for patients who would require total laryngectomy as a surgical approach. This trial randomly allocated patients to induction chemotherapy with cisplatin and 5-florouracil followed by definitive radiation versus primary surgical resection and postoperative radiation. With a median follow-up of 10 years, this trial demonstrated no significant difference in 5- or 10-year overall survival or progression-free survival. Of note, two thirds of living patients in the chemoradiotherapy arm were able to retain their larynx (43).
Long-Term Follow-Up
Regardless of whether patients undergo primary surgery or radiation therapy, there is value in close posttreatment surveillance by head and neck surgeon and radiation oncologist. During the first 6 months after treatment, patients should be followed every 4 to 6 weeks with clinical examination, including fiberoptic nasopharyngoscopy. Recommended guidelines include a follow-up visit every 1 to 3 months during the first year, every 2 to 4 months for the second year, every 4 to 6 months for years 3 through 5, and every 6 to 12 months thereafter. Additionally, if the patient received comprehensive head and neck radiation, the serum thyrotropin should be measured every 6 to 12 months. Imaging evaluation of the neck most commonly with CT or MRI scan are obtained at 3 to 6 month intervals during the first 2 years or as indicated based on clinical findings. Functional imaging with 18FDG-PET can sometimes prove valuable to help differentiate posttreatment fibrosis from persistent or recurrent disease.
A study by Hermans et al. (36) examined findings on CT scan of the neck 3 to 4 months following completion of radiation therapy for patients with larynx or hypopharynx cancer to examine correlation with long-term outcome. The authors suggest that in patients achieving complete radiographic resolution of all pretreatment disease, the likelihood of subsequent local failure is very small. These patients might therefore undergo routine clinical examination with repeat imaging reserved for instances where the clinical examination becomes suspicious for recurrence. For patients who achieved <50% reduction in tumor volume or retained a mass ≥1 cm on the posttreatment imaging study, the likelihood of local failure was 100% and 30%, respectively. In these patients, repeat CT at 3 to 4 months, FDG PET, or biopsy is therefore recommended. Preliminary reports indicate that the results of the first post-RT FDG-PET scan may be a strong predictor of developing locoregional disease recurrence (78).
In the posttreatment setting of hypopharynx cancer patients, the involvement of an experienced head and neck radiologist is highly desirable for optimal interpretation of imaging results. Soft-tissue changes following ablative surgery and reconstruction or following high-dose radiation or chemoradiation with resultant edema and fibrosis can be very difficult to differentiate from tumor, particularly for the inexperienced reader.
Management of Recurrence
After completion of treatment, patients should be followed closely for signs of recurrent or persistent disease. If recurrence is suspected, this should generally be confirmed by biopsy. If biopsy is confirmatory, then the patient should undergo complete restaging to assess the extent of disease. In the setting of local or regional disease alone, patients treated with initial radiation or chemoradiation can be considered for surgical salvage therapy. Although salvage surgery following comprehensive head and neck radiation and chemotherapy presents several resection and reconstructive healing challenges for the surgeon, selected patients may still derive long-term benefit from this approach. Recurrent patients who initially received comprehensive head and neck radiation have traditionally not been considered good candidates for repeat high-dose radiation in light of normal tissue tolerances. However, with the advent of highly conformal radiation delivery techniques, selected patients may benefit from reirradiation approaches in conjunction with systemic chemotherapy (77). Many patients with recurrent disease, however, are not good candidates for aggressive surgery or radiation salvage therapy and are best served with systemic chemotherapy and/or best supportive care approaches.
In the setting of distant metastatic disease, further treatment will focus on palliative goals. If the patient is experiencing significant local symptoms in the setting of asymptomatic distant metastases, palliative surgery or radiation may still warrant consideration. Most patients with distant metastatic disease and adequate performance status should be considered for systemic therapy and/or best supportive care options.
Quality of Life
Assessment of parameters including functional status, organ preservation, treatment cost, and patient-assessment of quality of life play an increasingly important role in the evaluation of overall treatment efficacy. For larynx and hypopharynx cancer patients, a focus of contemporary clinical investigation has been the study of treatments designed to preserve laryngeal function for patients traditionally treated with total laryngectomy. A frequently cited but somewhat controversial study by McNeil et al. (46) employed a questionnaire administered to healthy individuals and concluded that some might forgo total laryngectomy in favor of alternative therapy even if this choice diminished their ultimate chance for cure. A more recent report by El-Deiry et al. (26) evaluated long-term quality of life in a matched pair analysis comparing the surgical and nonsurgical treatment of patients with advanced head and neck cancer involving the oropharynx, hypopharynx, and larynx. Although patients in the surgery arm demonstrated worse speech outcomes than those treated with chemoradiation, this difference did not carry over to the overall quality of life score. These investigators concluded that, although it seems reasonable that organ preservation (nonsurgical) treatment will uniformly result in a higher quality of life, the complexities of human adjustment and multitude of potential treatment effects render this assumption invalid for many patients.
There have been relatively few prospective assessments of quality of life following treatment for head and neck cancer. In a subset of locally advanced patients requiring radical surgery such as total laryngectomy and partial pharyngectomy, the functional deficits are predictable. However, for patients undergoing “organ preservation” with radiation alone or in combination with chemotherapy, it can be difficult to assess the true extent and quality of organ preservation. Regardless of the primary treatment approach, these patients often require long-term speech, swallow, and dental rehabilitation. A study from Meyer et al. (49) retrospectively assessed speech intelligibility and quality of life in survivors of head and neck cancer. A total of 64 patients were enrolled; 31 underwent RT alone, five surgery alone, and 28 received both. All patients underwent comprehensive subjective and objective testing of speech function and quality of life. They found significant subjective and objective deficits in speech and quality of life even 5 years after completion of therapy. Terrell et al. (69) reported the results of a self-administered health survey of 570 patients at a Veteran's Administration hospital that demonstrated that the single most notable event having a negative impact on Quality of life was placement of a feeding tube. This was followed by medical comorbid conditions, presence of a tracheotomy tube, chemotherapy, and neck dissection.
A prospective study on quality of life utilizing the EORTC QLQ-C30 and QLQ-head and neck 35 questionnaires was conducted in Sweden on 357 patients. This study found that quality of life issues were significantly associated with the site of origin, with stage at diagnosis being the most important predictor. Additionally, patients with hypopharynx cancer exhibited the poorest quality of life (35). A study from the University of South Carolina compared swallow related quality of life after surgery or radiotherapy for head and neck cancer using a dysphagia risk factor survey, the M.D. Anderson Dysphagia Inventory (MDADI). They found significantly better scores on the emotional and functional components of the MDADI for patients undergoing chemoradiation compared to those undergoing surgery followed by radiation (34).
Conclusion
Patients with cancers of the hypopharynx commonly present with advanced disease associated with varying degrees of compromise in speech and/or swallow function. Many hypopharynx cancer patients also carry significant medical and social comorbidities. Typically, small T1–2 lesions can be managed with either primary radiation or surgery with similar clinical outcome. For intermediate stage disease that would require laryngopharyngectomy for the surgical approach, an increasingly preferred treatment option is combined chemoradiation that has demonstrated equivalence to immediate surgery in cancer survival, however, with improved organ preservation and functional outcome. For bulky hypopharynx tumors with significant airway compromise, laryngeal distortion, and cartilage destruction, it is generally best to proceed with definitive surgery with postoperative radiation or chemoradiation. Despite an aggressive approach in the overall management of hypopharynx cancer patients, ultimate cure rates remain quite poor. There are relatively few early stage patients, and for many advanced stage patients it is difficult to achieve long-term control. Even for those patients with excellent response to therapy, there exists a continuous risk for the development of second malignancies, particularly of the upper aerodigestive track with long-term follow-up. Posttreatment patients often require aggressive speech and swallow therapy to maximize their functional outcome. There is significant interest in the incorporation of molecular targeted therapies in combination with traditional cytotoxic therapy and radiation in an effort to improve outcomes.
Acknowledgments
We acknowledge the assistance of Lindell R. Gentry, M.D., Charles W. Hodge, M.D., and Wolfgang Tome, Ph.D. in image editing and collection and Gregory Allen, M.D., Ph.D. for reference editing.










Nessun commento:
Posta un commento